|
A diversity-oriented rhodamine library for widespectrum bactericidal agents with low inducible resistance against resistant pathogens |
|
The antibiotics is among the greatest medical invention of all time. The legendary discovery of Prontosil by Gerhard Domagk in 1930s has embarked the beginning of the antibacterial era. Not only it has saved his daughter, Hildegard, from a severe streptococcal infection, but also saved many others, among which included Franklin D. Roosevelt Jr., the president of the United States, from a grave strep sinus infection. Sir Alexander Fleming has been deeply indebted for his serendipic discovery of penicillin. Over the years, thousands of antibiotics have been developed and protected everyone from bacteria. It has been estimated that antibiotics have raised the average life expectancy of human being by over 10 years. Yet, antibiotics are not once-and-for-all type solution against bacteria. The use of antibiotics is inevitably accompanied by the emergency of antibiotic resistance. Once valuable antibiotics gradually become ineffective and withdraw from the pharmaceutical inventory. This was not a major issue before 1970s since scientists managed to keep coming up new antibiotics to replenish the pool. Situation started to change in the 1980s when the pace of antibiotic slowed down due to the diminishing generation of novel antibiotic scaffolds from the secondary metabolite of soil actinomycetes. The World Health Organization issued warning over the wide-spread drug-resistant pathogens and the stagnant antibiotic pipeline. Major countries including China, the US, the UK and many others have passed legislations to mitigate the abusive use of the remaining antibiotics and promote the development of novel antibiotic drugs.
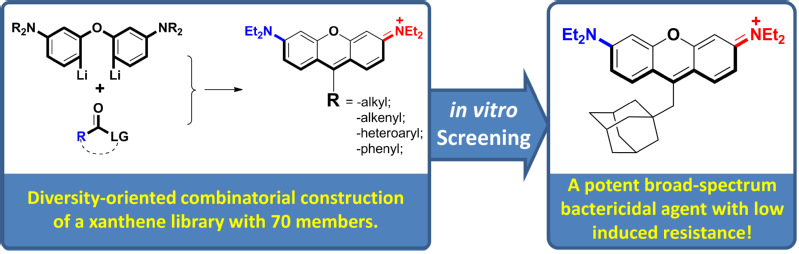
Recently, in collaborations with Prof. Daijie Chen of Shanghai Jiaotong University, we have discovered that a diversity-oriented rhodamine library is a new and rich molecular platform for new antibiotic hit compounds exhibiting potent bactericidal activity. Particularly, RD22 and RD53 are two antibacterial hits exhibiting favorable properties for further drug development. First, the growth of a wide-spectrum of Gram-positive and Gram-negative pathogens was inhibited by RD22 and RD53 with a low MIC. They are a potent bactericidal agent and a low dose at (2.5-10) × MIC could reduce the concentration of methicillin-resistant S. aureus, vancomycin-resistant E. faecalis, polymyxin E resistant A. baumannii by 4 orders of magnitude within ca. 4 hrs, more quickly than the current first-line and last-resort antibiotics, e.g. vancomycin, linezolid, tigecycline, and daptomycin. While the pathogens readily acquire resistance toward these existing antibiotics, exposing the cells toward sub-MIC concentration of RD22 and RD53 did not readily induce emergence of resistance. Fourth, they have a large therapeutic index of over 100, which is defined by the Lysis20/MIC. The work has recently published online by Nature Commun. (https://doi.org/10.1038/s41467-018-08241-3) with Xiao Luo of the Yang group as the first author. The work was financially supported by the National Natural Science Foundation of China (No. 21572061, 21822805). |
|
East China University of Science and Technology,School of Pharmacy
130 Meilong Road, Shanghai, China, 200237 Copyright By School of Pharmacy |