Chiral azepines, a class of seven-membered nitrogen-heterocycles, are privileged skeletons found in a great many of natural bioactive products and pharmaceuticals. In this context, the development of a straightforward and direct synthetic route to enantiomerically pure azepines has been an important research frontier and hot topic. Among the practicable approaches, [4 + 3] cyclization shows considerable superiority due to the high efficiency and atom economy. Nevertheless, developing novel catalytic asymmetric [4 + 3] cyclizations to achieve the structurally diverse azepines is still a challenging task in organic synthesis.
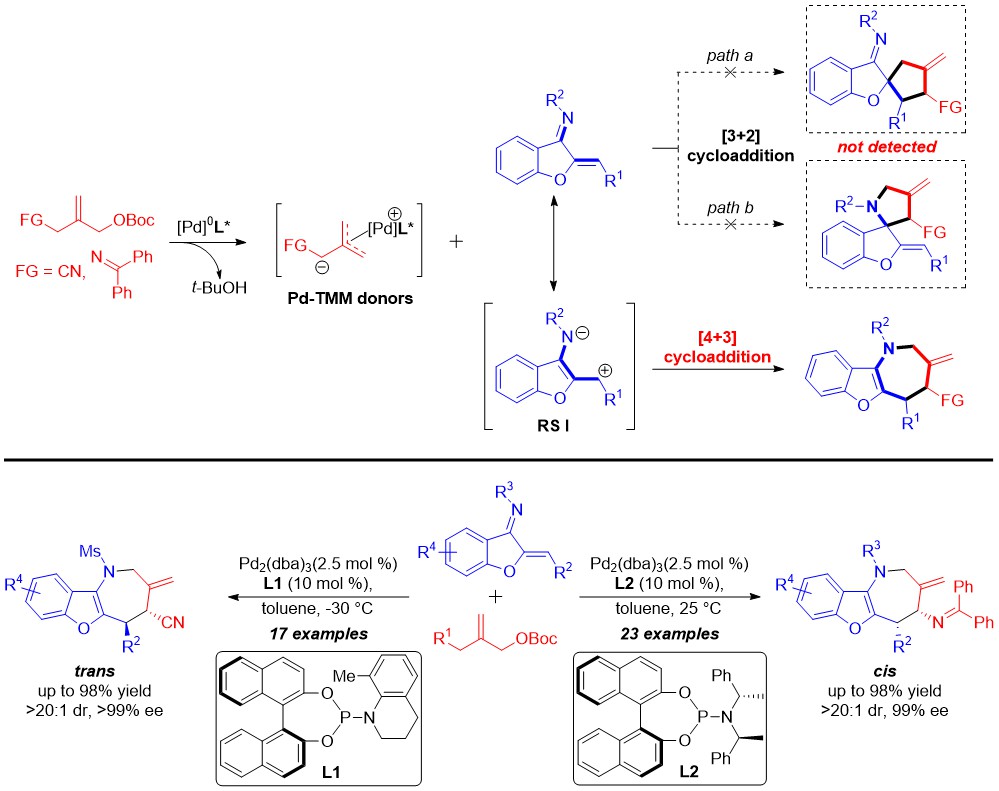
The group of Prof. Deng Wei-Ping reported a palladium-catalyzed asymmetric [4 + 3] cyclization of trimethylenemethane donors with benzofuran-derived azadienes, and the potential [3 + 2] side pathway was completely suppressed in the process. This protocol provides an efficient access to benzofuro[3,2-b]azepine frameworks in high yields (up to 98%) with exclusive regioselectivities and excellent stereoselectivities (up to >20:1 dr, >99% ee). This reaction represents the first catalytic asymmetric [4 + 3] cyclization of Pd-trimethylenemethane (Pd-TMM), which would enrich arsenal of Pd-TMM chemistry in organic synthesis. In addition, this strategy provides an alternative approach to chiral azepines via transition metal-catalyzed asymmetric [4 + 3] cyclization for the first time.
This work is supported by the National Natural Science Foundation of China (No. 21772038), the Fundamental Research Funds for the Central Universities, the China Postdoctoral Science Foundation (No. 2018M632037), and Shanghai Sailing Program (No. 18YF140560).
The authors:Yang-Zi Liu, Zhongao Wang, Zesheng Huang, Xing Zheng, Wu-Lin Yang,* and Wei-Ping Deng*
The link to this article:
https://onlinelibrary.wiley.com/doi/10.1002/anie.201909158.
