| Self-accelerating H2O2-responsive plasmonic nanovesicles for synergistic chemo/starving therapy of tumors |
|
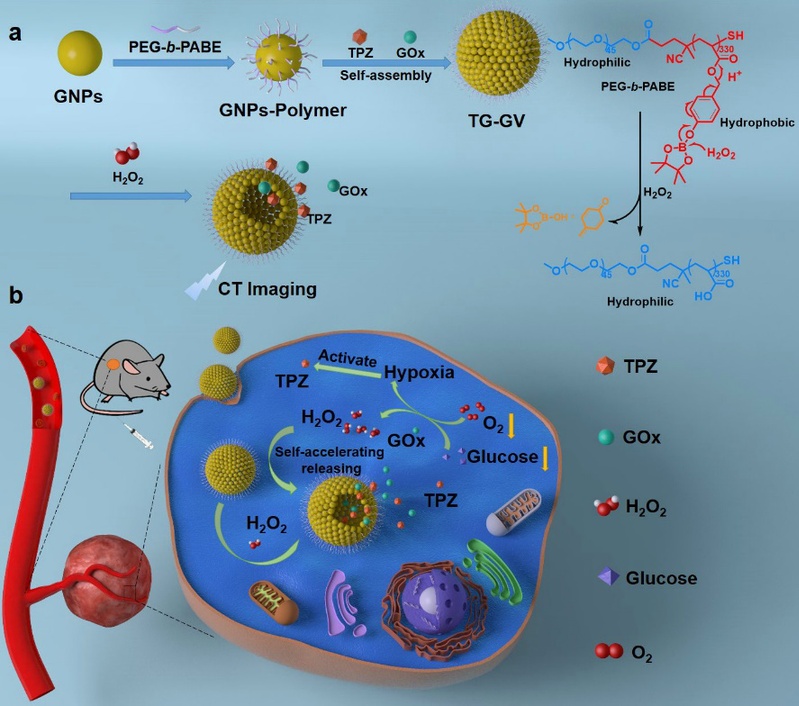
Recently, Professor Zhu Weiping in the School of Pharmacy of our university has developed a self-accelerating H2O2-responsive gold nanovesicle, which has realized the synergistic therapy of chemotherapy and starvation therapy on mice tumor. The related research results were published in the biomedical journal “Theranostics” under the title of “Self-accelerating H2O2-responsive Plasmonic Nanovesicles for Synergistic Chemo/starving therapy of Tumors” (Theranostics, 2020, 10 (19): 8691-8704). Organic-inorganic hybrid nanovesicles combine the high biocompatibility of organic materials and the unique optical, electrical, and magnetic properties of inorganic materials, and have ideal drug loading ability. In recent years, the hybrid nanovesicles have attracted more and more attention in the field of cancer diagnosis, treatment and imaging. Among them, gold nanovesicles with tunable absorption in the near-infrared range have achieved good diagnosis and treatment effect on tumors through photo-based imaging (photothermal/photoacoustic imaging) and therapy (photothermal/photodynamic therapy/light-controlled drug release). However, due to the limited penetration depth of light, the previously reported gold nanocapsules still face great challenges in the treatment of tumors in deep tissues. H2O2 is a by-product of many metabolic pathways in cells, mainly produced in mitochondria. The fast proliferation of tumor cells continuously produces H2O2 in large quantities due to mitochondrial dysfunction. In view of this characteristic, Professor Zhu Weiping and Professor Nie Zhihong (Fudan University) cooperated to design and prepare H2O2-responsive gold nanovesicle (TG-GVs). Both hypoxia pro-drug tirapazamine (TPZ) and glucose oxidase (GOx) are simultaneously encapsulated in the GVs during the assembly process to achieve specific drug release at tumor site.
In response to H2O2 in tumor, the TPZ- and GOx-loaded GVs (TG-GVs) dissociate to release payloads due to the oxidation of the hydrophobic phenyl boric ester groups of PEG-b-PABE into hydrophilic acrylic acid. The released GOx catalyzes the oxidation of glucose by oxygen in the tumor tissue to enhance the degree of hypoxia. As a result, the released TPZ molecules are activated to produce highly toxic free radicals in tumors for chemotherapy. In addition, GOx produces a lot of H2O2 during the oxidation of glucose, which further accelerates the dissociation of vesicles to speed up the release rate of cargoes, thus producing a self accelerating drug release effect. The TG-GVs shows significant tumor inhibition effects in 4T1 tumor-bearing mice, and the tumor growth inhibition rate was as high as 87.2%. It has been verified that chemotherapy and starvation treatment have produced synergistic therapeutic effects. This work breaks through the limitation that gold nanovesicles are only used in light controlled therapy in the past, and skillfully designs drug loaded nanovesicles with endogenous stimulus response by using the characteristics of tumor microenvironment. This nanoplatform broadens the application of gold nanovesicles and provides new ideas for the design and development of organic-inorganic hybrid nanovesicles. |
|
East China University of Science and Technology,School of Pharmacy
130 Meilong Road, Shanghai, China, 200237 Copyright By School of Pharmacy |