| Spatiotemporally controllable diphtheria toxin expression using a light-switchable transgene system combining multifunctional nanoparticle delivery system for targeted cancer therapy |
|
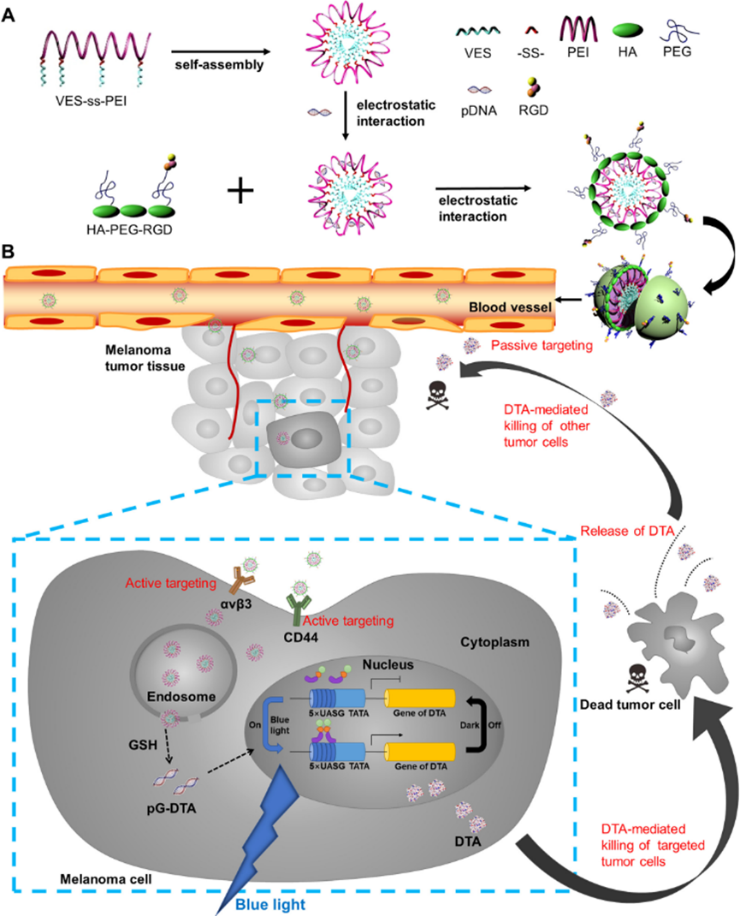
Professor Gao Feng’s and Professor Yang Yi’s research group (School of Pharmacy, East China University of Science and Technology) successfully developed a new nano drug delivery system, which could respond to the tumor microenvironment and release drug intelligently. Combined with the light-controlled gene expression system, the nano drug delivery system could control the expression of diphtheria toxin fragment A through light illumination and target melanoma and breast cancer. These results were published in Journal of controlled release and Acta Pharmaceutica Sinica B in 2020. Yang Yi's team developed a simple and efficient light controlled gene expression system (lightOn system) to realize the controllable expression of DTA with high toxicity on cells. To apply this system on tumor treatment, a nano drug delivery system was invented to overcome bio-obstacles, including penetrating tumor microenvironment, targeting and transfecting the plasmid to tumor cells. Based on these requirements, the Gao’s lab designed and constructed an intelligent nano drug delivery system with hyaluronidase (HAase) integrating, glutathione (GSH) reduction sensitivity with good in vivo stability. Synergize with lightOn system, the nano drug delivery system has been successfully applied to precise treatment of different kinds of cancer. In this study, we skillfully designed the GSH reduction sensitive part VES-ss-PEI as a core, which was a cationic amphiphilic gene loading micelle. Then, we developed a water-soluble fragment HA-PEG-RGD as a shell, which was able to target the newly generated endothelia cells at tumor site and cancer cells. The nanoparticles were administrated through intravenous injection to mice. Due to the EPR effect, the nanoparticles were passively accumulated to the tumor site. HA and RGD on the surface of the nanoparticles actively targeted CD44 receptor and αvβ3 integrin highly expressed on cancer cells. Then, the outer shell of the nanoparticles was degraded by hyaluronidase in the tumor to expose the inner core micelles. The core micelles entered the cytoplasm through lysosome escaping, degraded in high GSH concentration environment, release light-controlled diphtheria toxin plasmid (pGDTA) and effectively transfected cancer cells. After blue light irradiation, pGAVPO gene in pGDTA forms homodimer and combined with pu5 DTA gene and upstream UASG sequence to activate DTA expression, which killed the cancer Cells. The nanoparticles could also target the newly generated vascular at tumor site through RGD’s conjugation with highly expressed αvβ3 receptor, preventing neovascularization and inhibiting tumor metastasis. More importantly, the spacial and temporal expression of the lightOn system and the targeting ability of nano delivery system reduce the expression of DTA in normal tissues, maximizing its anti-cancer effect and minimizing the side effects.
Therefore, this multifunctional nanoparticles exhibited excellent antitumor properties by effectively utilizing a light-switchable gene expression system encapsulated in a nanoparticle delivery system, which may be a promising platform for treatment of cancer. |
|
East China University of Science and Technology,School of Pharmacy
130 Meilong Road, Shanghai, China, 200237 Copyright By School of Pharmacy |